[vc_row type=”in_container” full_screen_row_position=”middle” scene_position=”center” text_color=”dark” text_align=”left” overlay_strength=”0.3″ shape_divider_position=”bottom” bg_image_animation=”none”][vc_column column_padding=”no-extra-padding” column_padding_position=”all” background_color_opacity=”1″ background_hover_color_opacity=”1″ column_link_target=”_self” column_shadow=”none” column_border_radius=”none” width=”1/1″ tablet_width_inherit=”default” tablet_text_alignment=”default” phone_text_alignment=”default” column_border_width=”none” column_border_style=”solid” bg_image_animation=”none”][vc_column_text]
- Oral FASN inhibitor TVB-2640 significantly reduces liver fat with a 61% responder rate
- Demonstrates improvement in markers of liver function and fibrosis
- Results to be presented on August 28 at virtual EASL ILC 2020
San Mateo, California, June 17, 2020 – Sagimet Biosciences, a clinical-stage biotechnology company, announced today positive results from FASCINATE-1, the Phase 2 clinical trial of its oral, once-daily FASN inhibitor TVB-2640, the company’s lead product candidate that is currently being evaluated as a potential treatment for nonalcoholic steatohepatitis (NASH). The preliminary data showed that TVB-2640 significantly reduced liver fat, the primary efficacy endpoint of this trial, with a 61% responder rate in the 50 mg group. Participants also showed improvement in markers of liver function and fibrosis. As previously announced, the results will be shared in a virtual, late-breaker oral presentation scheduled for August 28 at 11:15 a.m. Central European time (2:15 a.m. Pacific time/5:15 a.m. Eastern time) at the upcoming European Association for the Study of the Liver (EASL) International Liver Congress™ 2020 (ILC).
“These results are extremely encouraging in light of the increasing global prevalence of NASH and a significant unmet medical need for safe and effective treatment options,” said Sagimet Chief Medical Officer Bill McCulloch. “We show that well-tolerated, oral TVB-2640 reduces liver fat, a major driver of NASH, and has the potential to be a foundational therapy for this disease either alone or in combination.”
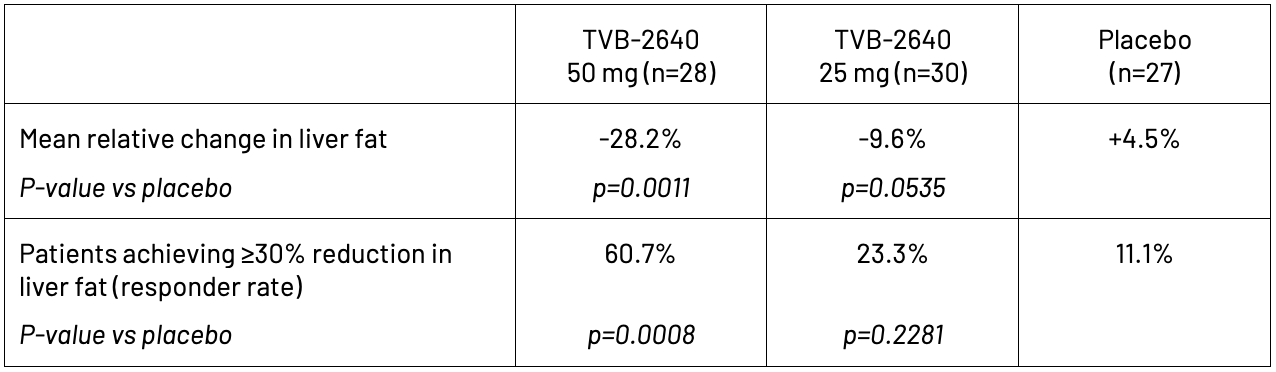
In the Phase 2 randomized, placebo-controlled trial of 99 NASH patients in the United States, clinicians evaluated the safety and efficacy of oral, once-daily dosing of TVB-2640 for 12 weeks. Study participants were required to have at least 8% liver fat at baseline, as measured by magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), and evidence of stage F1 to F3 liver fibrosis. The study demonstrated a statistically significant, dose-dependent relative reduction in liver fat of 28.2% in the 50 mg group versus an increase of 4.5% in the placebo group. TVB-2640 also significantly decreased ALT by up to 20.4% and LDL-cholesterol by up to 7.6% at week 12. These decreases indicate improved liver function and metabolic health.
TVB-2640 was well-tolerated with a benign adverse event profile, predominantly grade 1 events and no on-treatment serious adverse events.
“These promising clinical results are the first of their kind for a FASN inhibitor, showing a high response rate in liver fat reduction, which correlate with histological improvement as demonstrated in multiple other clinical trials,” said Rohit Loomba, M.D., director, NAFLD Research Center, University of California San Diego, and coordinating investigator of the study. “The strong effect and benign safety profile observed to date also indicate a solid foundation for advancing TVB-2640 into later stages of clinical development.”
An additional 50 mg cohort of 25-30 NASH patients in China has started screening. Further information about the Phase 2 study [NCT03938246] can be found at ClinicalTrials.gov.
About TVB-2640
TVB-2640 is an orally bioavailable, first-in-class fatty acid synthase (FASN) inhibitor. FASN is a key enzyme in the de novo lipogenesis (DNL) pathway that is responsible for the synthesis of excess fat in the liver of patients with NASH. Sagimet’s approach targets this key driver of NASH. The company announced the initiation of dosing in April 2019 in a randomized, placebo-controlled Phase 2 trial (FASCINATE-1), which evaluated the impact of TVB-2640 in 99 NASH patients in the United States. The primary efficacy endpoint was the impact of TVB-2640 on liver fat reduction by MRI-PDFF following 12 weeks of once-daily, continuous dosing. The trial also evaluated TVB-2640’s impact on levels of plasma lipids, liver enzymes, inflammatory and fibrotic biomarkers. The company has demonstrated in preclinical models that blocking FASN not only reduces liver fat, but directly reduces inflammation and fibrosis – addressing three major drivers of NASH.
About Sagimet
Sagimet Biosciences is a clinical-stage biopharmaceutical company focused on developing novel therapeutics to treat important diseases such as the liver disease NASH and specific cancers, with focus on targeting dysfunctional metabolic pathways. The company has unique expertise in FASN biology and has created a platform of proprietary FASN inhibitors. For more information, please visit www.sagimet.com.
###
Media Contact
Jamie Lacey-Moreira
+1 410-299-3310
jamielacey@presscommpr.com[/vc_column_text][/vc_column][/vc_row]

