Programs
Denifanstat – Our Lead Product Candidate in Multiple Indications
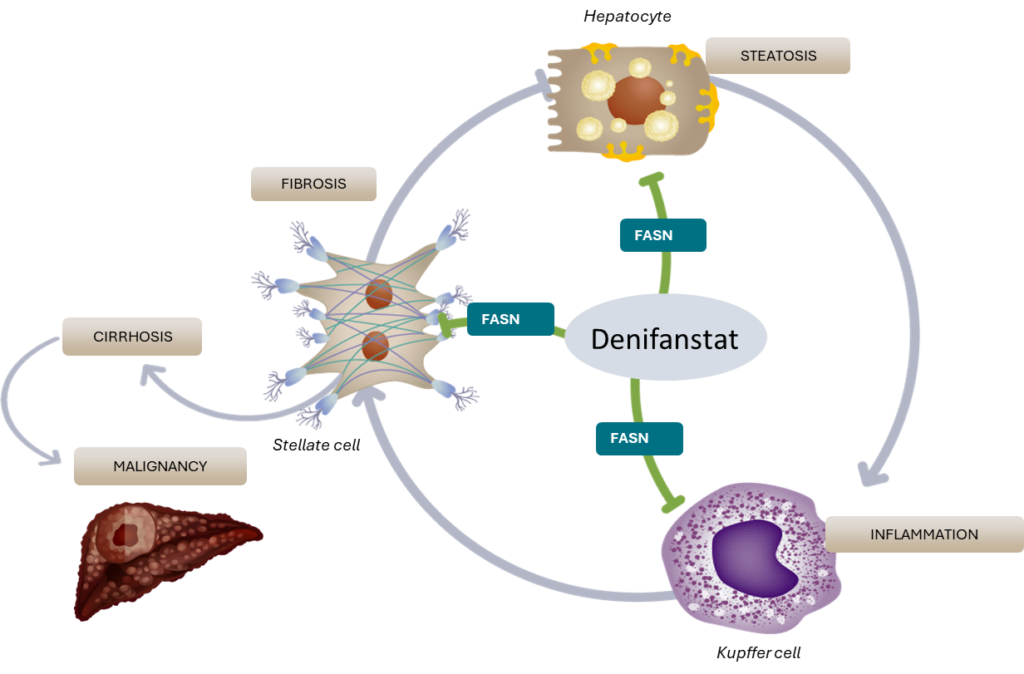
Denifanstat, our lead product candidate, is an oral, once daily pill and selective fatty acid synthase (FASN) inhibitor in development for the treatment of MASH. FASN is the key enzyme in the de novo lipogenesis (DNL) pathway that converts metabolites of dietary sugars such as fructose into palmitate, a saturated fatty acid.
Denifanstat was selected from our extensive compound library after a rigorous medicinal chemistry and preclinical development effort. We received Fast-Track designation for denifanstat from the FDA for the treatment of MASH in March 2021. In October 2024, the FDA granted Breakthrough Therapy designation to denifanstat for the treatment of noncirrhotic MASH with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis). Denifanstat has been studied in more than 740 subjects, including healthy volunteers and patients with MASH, acne and solid tumors.
Metabolic Dysfunction-Associated Steatohepatitis (MASH)
MASH is an aggressive form of fatty liver disease characterized by an abnormal build-up of excess fat in the liver, inflammation and fibrosis along with systemic metabolic changes including dyslipidemia and insulin resistance. Left untreated, the liver becomes cirrhotic and even cancerous with time. The damage can also exacerbate a spectrum of other health problems including cardiovascular diseases, obesity, type 2 diabetes and metabolic syndrome. MASH is a growing epidemic that affected more than 265 million people worldwide in 2019. Currently, there is only one recently approved treatment in the United States and no currently approved treatments in Europe.
We believe that denifanstat is differentiated among drug candidates in development for MASH due to its ability to directly target hepatocytes, inflammatory cells and stellate cells in the liver.
We announced positive topline results from FASCINATE-2, a Phase 2b clinical trial of denifanstat in MASH with liver biopsy-based primary endpoints, in January 2024. FASCINATE-2 was a Phase 2b clinical trial of denifanstat in biopsy-confirmed metabolic dysfunction-associated steatohepatitis (MASH) patients with moderate to advanced fibrosis (F2/F3) at week 52. In this trial, denifanstat, an oral, selective FASN inhibitor, showed statistically significant improvements relative to placebo on both of the primary endpoints of MASH resolution without worsening of fibrosis with ≥2-point reduction in NAS, and ≥2-point reduction in NAS without worsening of fibrosis. Denifanstat-treated patients also showed statistically significant fibrosis improvement by ≥ 1 stage with no worsening of MASH, and a greater proportion of MRI-derived proton density fat fraction (MRI-PDFF) ≥30% responders relative to placebo.
Combination Potential and Other MASH Indications
Based on its proposed mechanism of action, oral administration and tolerability profile to date, we believe denifanstat has the potential to be a backbone monotherapy as well as improve clinical activity in combination with a broad set of other drugs. We presented pre-clinical data at EASL in 2024 for two mouse models of MASH, showing that the combination of a FASN inhibitor (TVB-3664, a surrogate for denifanstat) and the thyroid hormone receptor beta (THRb) agonist, resmetirom, had a synergistic effect on important liver disease markers, including improvement of NAS by histologic analysis and more robust improvement in hepatic collagen content compared to the single agents. Synergistic activity of the combination was demonstrated in the rate of histological improvement (NAS ≥2 points). The FASN inhibitor monotherapy showed 33% improvement, resmetirom monotherapy showed 25% improvement, and the combination of the two showed an 80% improvement, a level of improvement that greatly exceeds a simple addition of the activity of the two drugs. We believe combination therapy has the potential to play a meaningful role in the MASH treatment paradigm to effectively address all patient segments.
In the second half of 2025, subject to consultation with regulatory authorities, we plan to initiate a Phase 1 clinical trial to evaluate the pharmacokinetics (PK) and tolerability of a combination of denifanstat and resmetirom with an anticipated data readout in the first half of 2026. We anticipate building on the outcome of this Phase 1 clinical PK trial, if positive, to develop a combination product for MASH patients.
Given the disease complexity as well as the heterogeneity and large size of the MASH patient population, we intend to study denifanstat in other MASH indications such as cirrhotic (F4) MASH and pediatric MASH to maximize its full clinical and commercial potential.
Acne
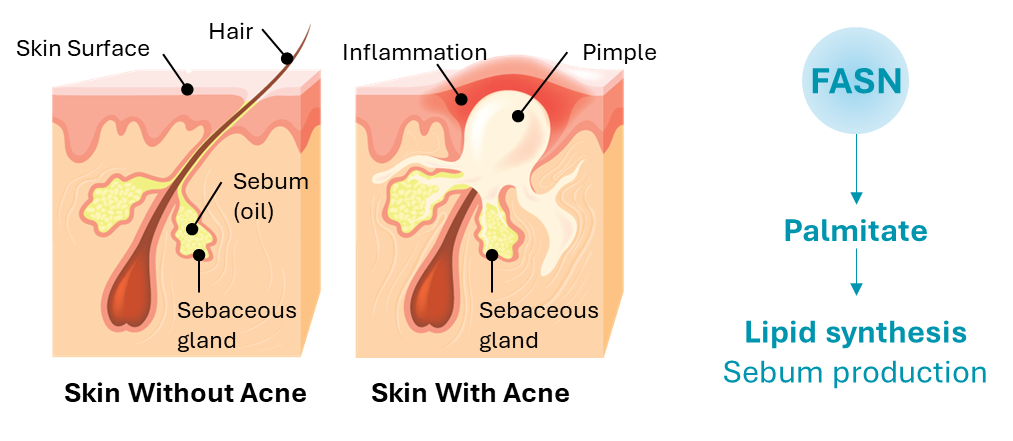
Acne is the most common skin condition in the United States, affecting up to 50 million Americans annually. Acne is a promising therapeutic area for application of FASN inhibitors because FASN is required for sebum production, which is upregulated in acne and leads to exacerbation of acne lesions including development of nodules and cysts. In two Phase 1 clinical studies, denifanstat reduced overall sebum production, including palmitate and sapienic acid lipids.
TVB-3567
Our second FASN inhibitor, TVB-3567, is a potent and selective small molecule FASN inhibitor, planned to enter clinical development for the treatment of acne. TVB-3567 showed potent FASN inhibitory activity based on inhibition of palmitate synthesis in human, rat, mouse, and dog cell lines; a single dose of TVB-3567 inhibited palmitate synthesis in a rat model. These studies include the standard suite of IND-enabling, GLP-compliant safety pharmacology and genotoxicity studies, and GLP-compliant general toxicology studies of up to four weeks treatment duration in rats and dogs.
In March 2025, we announced the clearance of our Investigational New Drug (IND) application for a first-in-human Phase 1 clinical trial of TVB-3567. Following the IND clearance, we initiated a first-in-human Phase 1 clinical trial of TVB-3567 for development of an acne indication in June 2025. The Phase 1 clinical trial is a randomized double-blind placebo-controlled trial designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of TVB-3567 in healthy participants with or without acne. The trial is expected to be comprised of several parts, including single ascending dose cohorts and multiple ascending dose cohorts in participants without acne, followed by testing in participants with acne including evaluation of pharmacodynamic biomarkers.
Phase 3 Clinical Trial in Acne
In June 2025, we reported that denifanstat met all primary and secondary endpoints in a Phase 3 clinical trial for the treatment of moderate to severe acne vulgaris conducted by Sagimet’s license partner Ascletis Bioscience Co. Ltd. (Ascletis) in China. Ascletis reported that denifanstat was generally well-tolerated. The Phase 3 clinical trial (NCT06192264) was a randomized, double-blind, placebo-controlled, multicenter clinical trial in China to evaluate the safety and efficacy of denifanstat for the treatment of patients with moderate to severe acne. The 480 enrolled patients were randomized 1:1 into two treatment arms to receive denifanstat 50mg or placebo, once daily for 12 weeks.
Oncology
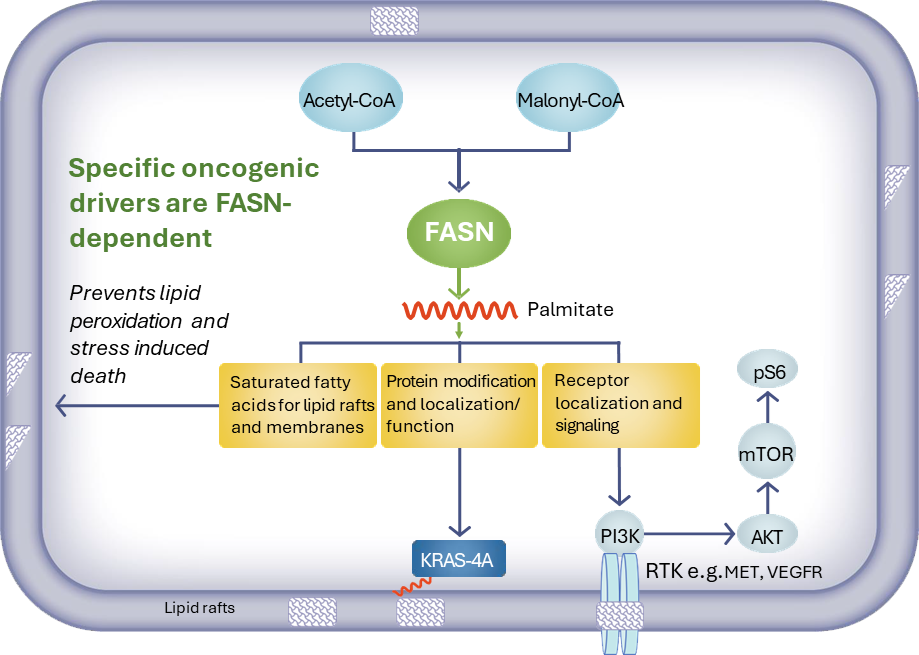
Dysregulation of lipid metabolism is a hallmark of certain cancers. Increased expression of FASN has been associated with poor prognosis and reduced survival in tumor cell types. Several cancer types have been shown to upregulate FASN to rewire lipid metabolism and change the nature of the tumor cell membrane making these cells resistant to traditional cancer drugs. FASN inhibition can also potentially address the enormous challenge of resistance to cancer therapies.
We completed a Phase 1 clinical trial with denifanstat in 136 patients with advanced, heavily pretreated and mostly metastatic solid tumors, which demonstrated clinical activity in defined patient populations and provides the foundation for future clinical development.
Our strategy is to evaluate denifanstat either alone or in combination with other classes of oncology drugs in specific subsets of solid tumors that are FASN-dependent.
These include:
- Glioblastoma – Denifanstat is currently being tested in a Phase 3 clinical trial in glioblastoma (GBM), by Ascletis, our license partner in China. In September 2023, Ascletis announced the enrollment of 120 recurrent GBM patients in its Phase 3 GBM trial.
- Metastatic castration resistant prostate cancer, FASN-dependent –– Investigator-sponsored Phase 1 clinical trial of denifanstat in combination with enzautamide therapy ongoing.
- Hepatocellular carcinoma FASN-dependent – Positive preclinical combination with kinase inhibitors, supported by translational bioinformatics.
- Non-small cell lung cancer/KRAS mutation – Positive preclinical combination results with KRAS inhibitor, encouraging results observed in patients with NSCLC KRASM tumors enrolled in a Phase 1 clinical trial in patients with solid tumors.