We are a clinical-stage biopharmaceutical company
developing novel fatty acid synthase (FASN) inhibitors that are designed to treat dysfunctional metabolic and fibrotic pathways in diseases resulting from the overproduction of the fatty acid, palmitate. We are exploring the use of our FASN inhibitors, which include our lead product candidate denifanstat and our pipeline product candidate, TVB-3567, in metabolic dysfunction associated steatohepatitis (MASH), acne and select forms of cancer, disease areas in which dysregulation of fatty acid metabolism plays a key role.
Denifanstat in MASH
Our lead product candidate, denifanstat, is an oral, once-daily pill and selective FASN inhibitor in development for the treatment of metabolic dysfunction-associated steatohepatitis (MASH), formerly known as nonalcoholic steatohepatitis (NASH). FASCINATE-1, our Phase 2 clinical trial in patients with MASH, showed that denifanstat was well-tolerated with a favorable pharmacokinetic profile. We announced the successful completion of FASCINATE-2, a Phase 2b clinical trial of denifanstat in MASH with liver biopsy-based primary endpoints, in January 2024. Denifanstat has been granted Breakthrough Therapy designation by the FDA for the treatment of non-cirrhotic MASH with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis), and end-of-Phase 2 interactions with the FDA have been successfully completed, supporting the advancement of denifanstat into further development.
Development Pipeline: Indications and Clinical Milestones
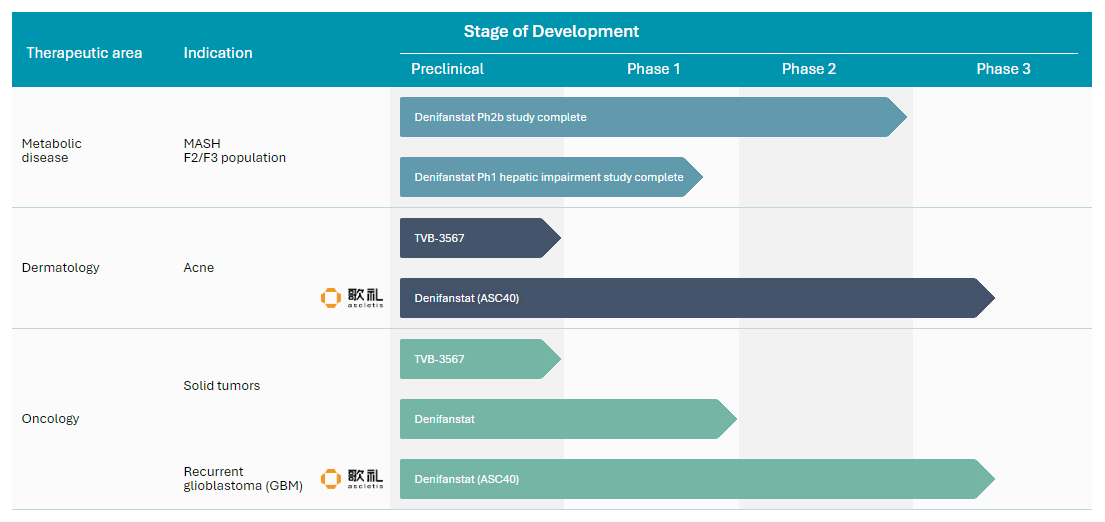
Stage of Development
Therapeutic area
Indication
Preclinical
Phase 1
Phase 2
Phase 3
* Trial conducted in China by Ascletis, who has licensed development and commercialization rights to all indications in Greater China


