Pipeline
Development Pipeline: Indications and Clinical Milestones
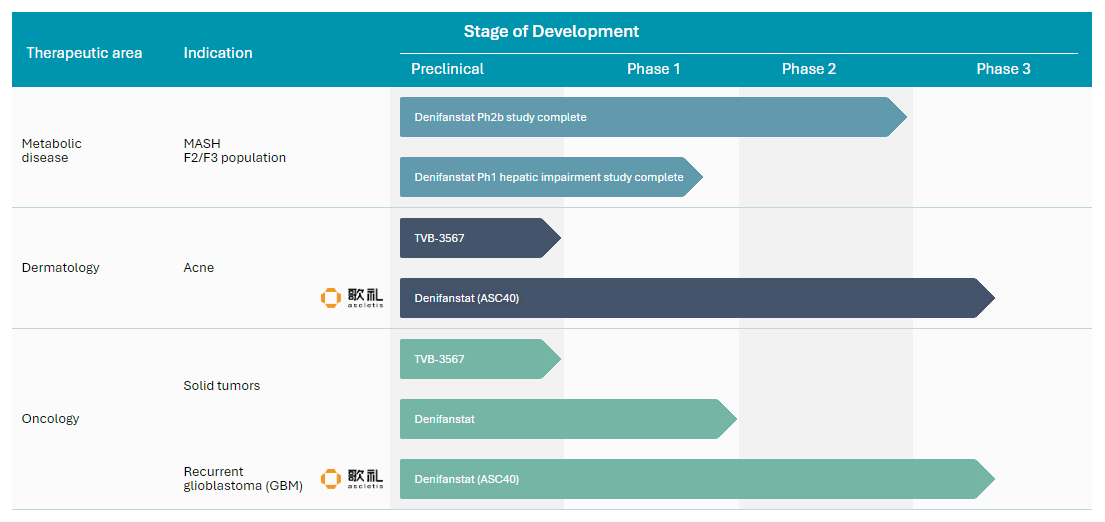
Stage of Development
Therapeutic area
Indication
Preclinical
Phase 1
Phase 2
Phase 3
Denifanstat Phase 3 F2/F3 ready
TVB-3567
TVB-3567
* Trials conducted in China by Ascletis, who has licensed development and commercialization rights to all indications in Greater China
Development Pipeline: Indications and Clinical Milestones
Stage of Development
Therapeutic area
Indication
Expected Milestone / Status
Preclinical
Phase 1
Phase 2
Phase 3
Metabolic
disease
Denifanstat
Dermatology
Denifanstat (ASC40)
TVB-3567
* Trials conducted in China by Ascletis, who has licensed development and commercialization rights to all indications in Greater China


